Abstract
Introduction . Polycythemia Vera (PV) is a myeloproliferative neoplasm (MPN) whose major features are peripheral erythrocytosis and increased thrombotic risk, affecting morbidity and mortality. Essential Thrombocythemia (ET), a MPN characterized by high platelet generation rate, has been shown to be associated with a reduction in von Willebrand factor (vWf) large multimers and activity which depends on platelet turnover (Lancellotti et al, JTH 2015;13:1226-37). The acquired vWf type 2A defect in ET is thought to influence bleeding rate. Data on vWf and on a possible acquired von Willebrand deficiency in PV are less clear. Previous studies included PV patients with marked thrombocytosis, with bleeding history or included treated and untreated patients, with heterogeneous results. Therefore, the phenotypic features of vWf in PV patients with well-controlled haematocrit, routinely accessing the outpatient clinics remain largely undefined. In addition, whether the acquired vWf defect is similar between PV and ET remains poorly defined.
Methods . This is an observational, cross sectional study on consecutive PV patients diagnosed according to the 2008 WHO criteria, treated to keep the hematocrit <45% and on antithrombotic prophylaxis with low-dose aspirin, according to current recommendations. VWF:antigen (VWF:ag) and WVF:activity (VWF:act) were measured by chemiluminescence assays (Instrumentation Laboratory, Milan, Italy). Routine hematochemistry, genotype, clinical characteristics and medications were also recorded. To compare vWF-associated phenotype in PV and ET, we selected age- and sex-matched ET patients from a cohort of 69 patients previously-published from our group (Lancellotti et al., JTH 2015; 13:1226-37). PV and ET plasma samples were measured in the same laboratory with the same methods.
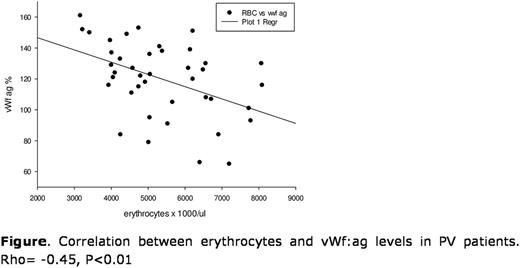
Results . Forty-one consecutive PV patients gave their written consent (age 66±11 years, 32% females). Mean (±SD) haematologic values were: hematocrit 43.9±2.9%, leukocytes 9.5±4.6x103/µl, platelets 353±260x103/µl. vWf:ag levels averaged 120±24%, vWf:act 115±21%, the act/ag ratio was 0.96±0.06. VWf:ag and vWf:act were significantly correlated (rho=0.98, p<0.001). On univariate analysis, both vWf:ag and vWf:act significantly and positively correlated with male sex, blood groups (higher in non-0 vs. 0 blood groups), hydroxyurea treatment, JAK2V617F allelic burden, and inversely correlated with erythrocytes and platelets. Multivariable analysis confirmed erythrocyte count (inversely, p<0.001, Figure), blood group (p=0.01) and JAK2V617F allele burden (p=0.02) as significant predictors of vWf:ag (adj Rsqr for the entire model=0.54). Erythrocyte count (inversely, p<0.001) and blood group (p=0.02) independently predicted vWf:Act as well (adj Rsqr for the entire model=0.49).
We then compared vWf:ag and vWf:act between these PV patients and 41 ET patients (age 64±11 years p=0.4 vs. PV patients, 38% females). As compared to ET patients, PV patients showed similar levels of vWf:ag (120±23 and 136±57% in PV and ET, respectively; p=0.4), but significantly (p<0.01) higher levels of vWf:act (114±20 and 86±47%, in PV and ET, respectively) and act/ag ratios (0.9±0.06 and 0.6±0.2, in PV and ET, respectively).
Conclusions. PV patients treated according to current recommendations do not show evidence for acquired vWf defect. In PV patients vWf levels seem dependent on erythrocyte count (inversely), blood groups (as in the normal population) and JAK2V617F allelic burden, with features different from age- and sex-matched ET patients. Future studies are needed to investigate the pathophysiological mechanisms linking eytrhocytes and vWf levels in PV.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal